Answer:
Reaction goes through
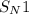 mechanism.
mechanism.
Step-by-step explanation:
Solvolysis of tert-butyl bromide in methanol gives methyl tert-butyl ether as major product obtained from nucleophilic substitution of bromine atom by methanol.
Reaction mechanism goes through
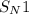 mechanism.
mechanism.
In the first step, a tert-butyl carbocation is produced. This is the rate limiting step.
In the second step, methanol attacks the carbocation and gets bind to it through a covalent bond.
In the third step, deperotonation occurs to give methyl tert-butyl ether.
Reaction mechanism has been shown below.