Answer : The Lewis structure of
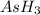 shows 1 non-bonding electron pair on as.
shows 1 non-bonding electron pair on as.
Explanation :
Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
In the Lewis-dot structure the valance electrons are shown by 'dot'.
The given molecule is,
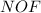
As we know that nitrogen has '5' valence electrons, oxygen has '6' valence electrons and fluorine has '7' valence electron.
Therefore, the total number of valence electrons in
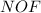 = 5 + 6 + 7 = 18
= 5 + 6 + 7 = 18
According to Lewis-dot structure, there are 6 number of bonding electrons and 12 number of non-bonding electrons.
The Lewis-dot structure of
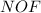 is shown below.
is shown below.