Answer:
514.76 grams of carbon tetrachloride are required.
Step-by-step explanation:
P = Pressure of vapor = 1.77 atm
V = Volume of vapor =
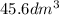 = 45.6 L
= 45.6 L
n = number of moles of Freon-21 = ?
R = Gas constant = 0.0821 L.atm/mol.K
T = Temperature of vapor = 21°C = 294.15 K
PV = nRT( ideal gas equation)
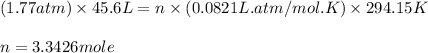
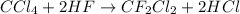
Moles of Freon-21 = 3.3426 mol
According to reaction, 1 mol of Freon-21 is obtained from 1 mole of carbon tetrachloride.
Then 3.3426 moles of Freon-21 will be obtained from:
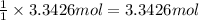
Mass of 3.3426 moles of carbon tetrachloride:
3.3426 mol × 154 g/mol = 514.76 g
514.76 grams of carbon tetrachloride are required.