Answer:
3h12min
Step-by-step explanation:
We need to apply our thermodynamics concepts to find the required time in which the ice will melt at a given temperature.
In our data we have defined that,
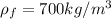
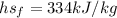
Performing an energy balance you have to:
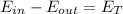
Internal energy is given by,
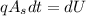
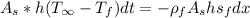
Integrating,
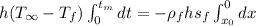
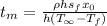
Replacing the values,
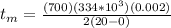
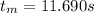
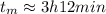
Note: It was assumed that fros is isothermal and the radiation exchange is also negligible.