Answer:
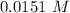
Step-by-step explanation:
The reaction between
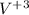 and EDTA would be:
and EDTA would be:
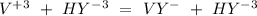
The EDTA in excess (
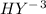 ) would react with the
) would react with the
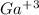 :
:
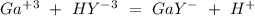
All the reactions have a 1:1 ratio. So:
Total moles of EDTA:
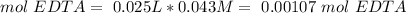
Moles that react with
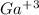 :
:
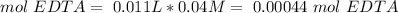
Moles that react with
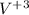 :
:
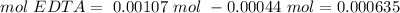
If we have a 1:1 ratio:
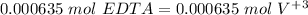
Now we can calculate the concentration:
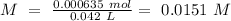 .
.