Answer:
(a)
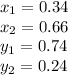
(b)
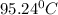
Step-by-step explanation:
Hello,
The Rachford-Rice equation should be used in this case:
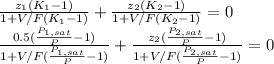
The distribution coefficients could be approximated via Raoult's law and the Antoine equation for the vapor pressure:
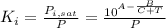
For n-hexane: A=6,9895, B=1216,92 and C=227,451
For n-octane: A=7,14462, B=1498,96 and C=225,874
*Those values and the Antoine law are used in °C and mmHg.
Replacing each value in the Rachford-Rice equation and solving for T, one gets the flash drum temperature as 95.24°C (attached picture), thus, the compositions in both the liquid and the vapor phases are:
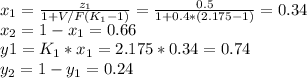
Best regards.