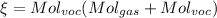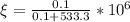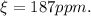Answer:
187ppm.
Step-by-step explanation:
To develop the problem we first need to perform mass conversions to moles. In this way for gases your conversion is given by,
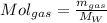
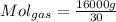
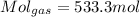
For the VOC's same:
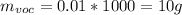
then,
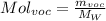
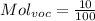
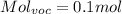
We only need to obtain the fraction of Voc's in exhaust. This will be in particles per million, so