Answer:
C) The atomic number increases by 1 and the mass remains the same.
Step-by-step explanation:
As per nuclear decay we know that in beta decay we have
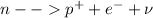
now we have
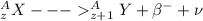
so here we can see that due to excess neutrons inside the nucleus it is unstable and then due to decay of one neutron one proton and electron
so here the electron ejected from the nucleus is known as beta particles
so as per above equation we know that correct answer will be
C) The atomic number increases by 1 and the mass remains the same.