Answer: Copper (I) iodide will precipitate first.
Step-by-step explanation:
We are given:
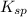 of CuCl =
of CuCl =
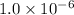
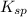 of CuI =
of CuI =
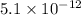
Concentration of
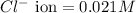
Concentration of
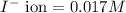
Solubility product is defined as the product of concentration of ions present in a solution each raised to the power its stoichiometric ratio.
![K_(sp)=[Cu^+][Cl^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/63hyvaqzxq92bdxlcmlj77ztdf5tbjwj1g.png)
Putting values in above equation, we get:
![1.0* 10^(-6)=[Cu^+]* 0.021](https://img.qammunity.org/2020/formulas/chemistry/high-school/m7qobtvx3u5n1pfpszlsruramglpsut30c.png)
![[Cu^+]=(1.0* 10^(-6))/(0.021)=4.76* 10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/jgxjc2iew1318kq738fhnt93fsawh6w6dv.png)
Concentration of copper (I) ion =
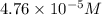
![K_(sp)=[Cu^+][I^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/4pu84jvspwhp3aygpb1bf7ovrs902mgsqj.png)
Putting values in above equation, we get:
![5.1* 10^(-12)=[Cu^+]* 0.017](https://img.qammunity.org/2020/formulas/chemistry/high-school/sbbl1yqpiv0b0uydbgy2uzxztjkc68ircs.png)
![[Cu^+]=(5.1* 10^(-12))/(0.017)=3.00* 10^(-10)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/8lxq3tobytfzua2mrs765v4hmkavpt4jr9.png)
Concentration of copper (I) ion =
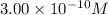
For the precipitation of copper (I) ions, we need less concentration of copper (I) ions. So, copper (I) iodide will precipitate first.