Answer:
2.0 moles
Step-by-step explanation:
Limiting reagent is the one which is present in small amount. The formation of the product is governed by the limiting reagent.
Given that:-
2.0 moles of A (with an excess of B) can make a maximum of 2.0 moles of C 3.0 moles of B (with an excess of A) can make a maximum of 4.0 moles of C
Thus,
Moles of C = moles of A = (4/3) moles of B
The balanced reaction may be:-
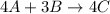
Given, moles of A = 2.0 moles
Moles of B = 3.0 moles
4 mole of A react with 3 moles of B
1 mole of A react with 3/4 moles of B
2 moles of A react with (3/4)*2 moles of B
Moles of B = 2.5 moles
Available moles of B = 3.0 moles
B is in excess. Thus, A is the limiting reagent.
4 mole of A produces 4 mole of C
Thus, C produced from 2 moles of A = 2.0 moles