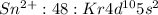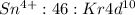Answer:
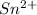 and
and
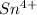
Explanation:-
Electronic configuration represents the total number of electrons that a neutral element contains. We add all the superscripts to know the number of electrons in an atom.
The electrons are filled according to Afbau's rule in order of increasing energies and thus the electronic configuration in terms of nearest noble gas for tin with atomic number of 50 and thus 50 electrons
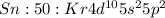
It can lose two electrons from 5 p orbital to form
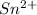 or can lose 4 electrons from 5p and 5s to form
or can lose 4 electrons from 5p and 5s to form
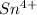 .Tin loses electrons to acquire noble gas configurations to acquire stability.
.Tin loses electrons to acquire noble gas configurations to acquire stability.