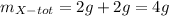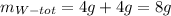Answer:
There are required 8 g of W and 4 g of X
Step-by-step explanation:
Hi, first this is what we know:
a) W, X and Y are used in a 2:1:3 ratio to produce Z, meaning this that for each gram of X used, 2 grams of W and 3 of Y are required.
b) Also, for each gram of X: 1 g X + 2 g W + 3 g Y= 6 g Z
Being that said, to produce 12 g of Z:
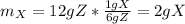
Following the ratio described in a) for the 12 g of Z 4 g W and 6 g Y are required.
On the other hand, to make those 6 g Y you required 4 g of W and 2 g of X
So, the total mass of W and X is :