Answer:
79.4 kg
Step-by-step explanation:
The formula for potassium iodide is:-
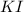
This means that they are present in the same mole ration, i.e. 1 : 1
Also for 2 samples, the ratio of the masses must be equal .
So,

Thus,
Mass of potassium in sample 1 = 13.0 g
Mass of Iodine in sample 1 = 42.3 g
Mass of potassium in sample 2 = 24.4 kg
Applying in the above formula, we get that:-
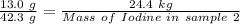
Mass of iodine in sample 2 = 79.4 kg