Answer:
0.07 moles of
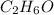 are left over
are left over
Step-by-step explanation:
1. Write down the balanced chemical equation for the combustion of ethanol with oxygen:
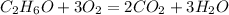
2. Find the limiting reagent to know which reagent is left over:
To find the limiting reagent take the number of moles given by the problem and divide this number between the stoichiometric coefficient from the balanced chemical equation. The smallest number will be the limiting reagent.
- For the ethanol :
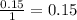
- For the oxygen:
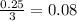
Therefore, the oxygen is the limiting reagent, that is the ethanol is the compound that will be left over.
3. Find the moles of ethanol left over.
- First calculate how many moles of ethanol reacted with the oxygen:
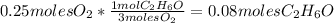
So 0.08 moles of
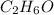 reacted with the oxygen.
reacted with the oxygen.
- Then subtract the moles of ethanol that reacted with the oxygen from the total moles of ethanol in the reaction:
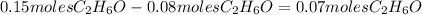 are left over.
are left over.