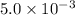Answer : The equilibrium constant for the reaction is
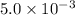
Explanation :
The given equilibrium reaction is:
![CuBr(s)+Br^-(aq)\rightleftharpoons [CuBr_2]^-(aq)](https://img.qammunity.org/2020/formulas/chemistry/college/wfquwbml983zcda97s3ngn1ftiisf39v07.png)
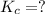
The dissociation reaction will be:
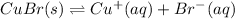
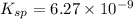
The formation reaction will be:
![Cu^+(aq)+2Br^-(aq)\rightleftharpoons [CuBr_2]^-(aq)](https://img.qammunity.org/2020/formulas/chemistry/college/o2xaus84eq0lp7thf31vlbx7fkxca5a8vq.png)
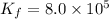
Thus, the value of equilibrium constant will be:
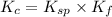
Now put all the given values in this expression, we get:
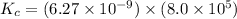
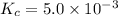
Therefore, the equilibrium constant for the reaction is