The correct number of significant figures : 17
Further explanation
Given
mass = 8.64 g
volume = 2 ml
Required
The density of liquid
Solution
Density is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
Density formula:
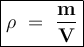
The density :
ρ = 8.64 : 2
ρ = 4.32 g/ml
If we want to find density, then the steps should be as above
But if we ignore the density and just want to find the multiplication value to clarify the significant figure, then we must pay attention to the rule of multiplication, that is, the end result of the multiplication has the least significant number of the numbers involved in multiplying.
8.64 = 3 sig fig
2.0 = 2 sig fig
then the product contains 2 sig fig
8.64 x 2.0 = 17.28 rounded to 2 sig fig ⇒ 17