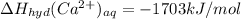Answer:
Heat of hydration of calcium ion is -1703 kJ/mol
Step-by-step explanation:
Dissolution equilibrium of calcium chloride:
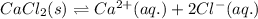
![\Delta H_(sol)=[1mol* \Delta H_(hyd)(Ca^(2+))_(aq.)]+[2mol* \Delta H_(hyd)(Cl^(-))_(aq.)]-[1mol* U(CaCl_(2))_(s)]](https://img.qammunity.org/2020/formulas/chemistry/college/3da9vac875fitetxnaz6me5xmicvkek2bg.png)
Where
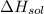 is heat of solution,
is heat of solution,
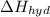 is heat of hydration and U represents lattice energy.
is heat of hydration and U represents lattice energy.
Here,
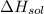 = -121 kJ/mol,
= -121 kJ/mol,
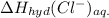 = -338 kJ/mol and
= -338 kJ/mol and
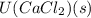 = -2258 kJ/mol
= -2258 kJ/mol
So, plug-in all the given values in the above equation-
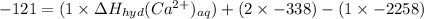
So,