Answer: The enthalpy change of the reaction is 239.2 kJ/mol
Step-by-step explanation:
- To calculate the mass of solution, we use the equation:
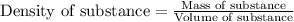
Density of solution = 1.0 g/mL
Volume of solution = 52.4 mL
Putting values in above equation, we get:
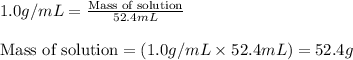
- To calculate the heat absorbed we use the equation:
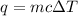
where,
q = heat absorbed = ?
m = mass of solution = 52.4 g
c = specific heat capacity of solution = 4.18 J/g.°C
 = change in temperature =
= change in temperature =
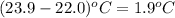
Putting values in above equation, we get:
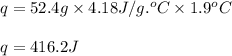
Heat absorbed by the solution = heat released by the reaction
- To calculate the number of moles, we use the equation:
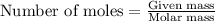
Given mass of zinc = 0.114 g
Molar mass of zinc = 65.4 g/mol
Putting values in above equation, we get:
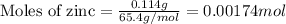
- Calculating the amount of heat released per mole of zinc, we get:

Conversion factor: 1 kJ = 1000 J
Hence, the enthalpy change of the reaction is 239.2 kJ/mol