Answer:
-88.66 kJ/mol
Step-by-step explanation:
The expressions of heat capacity (Cp,m) for C(s) and for H₂(g) are:
C(s): Cp,m/(J K-1 mol-1) = 16.86 + (4.77T/10³) - (8.54x10⁵/T²)
H₂(g): Cp,m/(J K-1 mol-1) = 27.28 + (3.26T/10³) + (0.50x10⁵/T²)
Cp = A + BT + CT⁻²
For the Kirchoff's Law:
ΔHf = ΔH°f +
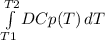
Where ΔH°f is the enthalpy at 298 K, T1 is 298 K, T2 is the temperature given (373 K), and DCp is the variation of Cp (products less reactants). ΔH°f for ethene is -84.68 kJ/mol and the reaction is:
2C(s) + 3H₂(g) → C₂H₆
So, DCp:
dA = A(C₂H₆) - [2xA(C) + 3xA(H₂)] = 14.73 - [2x16.86 + 3x27.28] = -100.83
dB = B(C₂H₆) - [2xB(C) + 3xB(H₂)] = 0.1272 - [2x4.77x10⁻³ + 3x3.26x10⁻³] = 0.10788
dC = C(C₂H₆) - [2xC(C) + 3xC(H₂)] = 0 - (2x(-8.54x10⁵) + 3x0.50x10⁵) = 15.58x10⁵
dCp = -100.83 + 0.10788T + 15.58x10⁵T⁻²
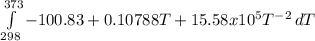 = -3796.48 J/mol = -3.80 kJ/mol (solved by a graphic calculator)
= -3796.48 J/mol = -3.80 kJ/mol (solved by a graphic calculator)
ΔHf = -84.68 - 3.80
ΔHf = -88.66 kJ/mol