Answer:
mass AsCl₃ in 8.2x10⁵ L = 99.2gAsCl₃
Step-by-step explanation:
Let's remember that 0.05 ppm (parts per million) would be write terms of mg/L. We have 0.05 mg/L, that is a concentration unit.
First of all we need to find the g/L of AsCl₃ present in water.
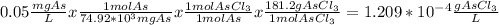
Now, we just need to multiply it by 8.2 x 10⁵ L to determine the mass of AsCl₃ present in that volume.
Finally we have:
 .
.
Have a nice day!