Answer:
Charge of 1 mole of electrons = -96488.46 C
Charge of 1 mole of protons/ hydrogen ions = +96488.46 C
Step-by-step explanation:
Given, Avogadro constant:-
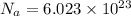
Thus, charge on 1 mole of electron can be calculated as:-
Charge on 1 electron =
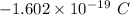
Charge on
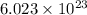 electrons =
electrons =
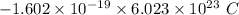 = -96488.46 C
= -96488.46 C
Charge of 1 mole of electrons = -96488.46 C
Charge of electron = Charge on proton (magnitude), Only the charge is different. Electron has negative charge and protons have positive charge.
Charge of 1 mole of protons/ hydrogen ions = +96488.46 C