Answer:
The volume of bubble is 5.13 cm³.
Step-by-step explanation:
Given that,
Volume of air bubble = 1.9 cm³
Gauge pressure = 1.7 atm
Suppose we find the volume of the bubble as it reaches the surface
We need to calculate the pressure at bottom
Pressure at bottom=gauge pressure+atmospheric pressure
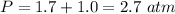
We need to calculate the volume of bubble
Using Boyle's law
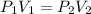
Put the value into the formula
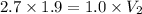
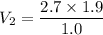
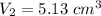
Hence, The volume of bubble is 5.13 cm³.