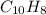Step-by-step explanation:
Molecular formulas is the actual number of atoms of each element in the compound while empirical formulas is the simplest or reduced ratio of the elements in the compound.
Thus,
Molecular mass = n × Empirical mass
Where, n is any positive number from 1, 2, 3...
For example:-
The empirical formula of naphthalene =
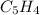
The molecular formula of naphthalene =