Answer:
0.00034% is the (w/w) concentration of sodium in 28 grams of bread.
Step-by-step explanation:
Mass of bread = 28 g =0.028 kg (1 g = 0.001 kg)
The ppm concentration of sodium = 3.4
1 ppm = 1 mg/kg
The ppm concentration of sodium = 3.4 mg/kg
This means 3.4 mg of sodium is present in 1 kg of bread.
The mass of sodium present in 0.028 kilograms of bread will be:
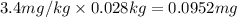
Mass of sodium present in bread = 0.0952 mg
Mass of bread in milligrams = 28 g = 28000 mg
w/w % : The percentage mass is the mass of the of solute present in total mass of the solution.
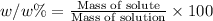
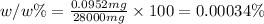
0.00034% is the (w/w) concentration of sodium in 28 grams of bread.