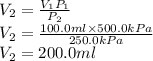Answer:
The final volume of the gas is 200.0 ml
Explanation:
Boyle's law is one of the gas laws that relates the volume and pressure of a certain amount of gas kept at a constant temperature.
Mathematically it can be expressed like this:
PV=k
where k is constant if the temperature and mass of the gas remain constant.
We can solve the problem by using this formula:
P₁V₁=P₂V₂
where
P₁= 500.0 kPa
V₁= 100.0 ml
P₂= 250.0 kPa
V₂ is unknown
Hence the final volume would be: