Answer:
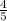 moles
moles
Step-by-step explanation:
The reaction equation is given as:
4NH₃ + 5O₂ → 4NO + 6H₂O
The number of moles of O₂ that completely reacted is given as 1 mole
To solve this problem, we are going to use a stoichiometric approach from the balanced reaction equation:
5 moles of O₂ will react completely to produce 4 moles of NO
1 mole of O₂ will therefore react to produce x mole of NO
5x = 4
x =
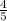 moles of NO
moles of NO