Answer:
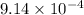 moles
moles
Step-by-step explanation:
We are given:
Vapor pressure of water = 19.8 torr
Total vapor pressure = 752 torr
Vapor pressure of oxygen gas = Total vapor pressure - Vapor pressure of water = (752 - 19.8) torr = 732.2 torr
To calculate the amount of oxygen gas collected, we use the equation given by ideal gas which follows:

where,
P = pressure of the gas = 732.2 torr
The conversion of P(torr) to P(atm) is shown below:
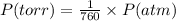
So,
Pressure = 732.2 / 760 atm = 0.9634 atm
V = Volume of the gas = 23 mL = 0.023 L
T = Temperature of the gas =
![22^oC=[22+273]K=295K](https://img.qammunity.org/2020/formulas/chemistry/high-school/lb4pbmgp68m814qvand69yw9b5bkw29dvx.png)
R = Gas constant =
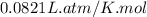
n = number of moles of oxygen gas = ?
Applying the equation as:
0.9634 atm × 0.023 L = n × 0.0821 L.atm/K.mol × 295.15 K
⇒n =
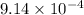 moles
moles