Answer:
P = 0.75 atm
Step-by-step explanation:
Using Boyle's law
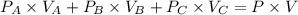
Given ,
For Bulb A
Pressure = 190 torr
The conversion of P(torr) to P(atm) is shown below:
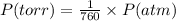
So,
Pressure = 190 / 760 atm = 0.25 atm
Volume = 200 mL
For Bulb B
Pressure = 1 atm
Volume = 400 mL
For Bulb C
Volume = 1.00 L = 1000 mL
Pressure = 75.994 kPa
The expression for the conversion of pressure in KiloPascal to pressure in atm is shown below:
P (kPa) =
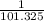 P (atm)
P (atm)
75.994 kPa =
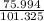 atm
atm
Pressure = 0.75 atm
Also, Total volume, V = 200 + 400 + 1000 mL = 1600 mL
Using above equation as:
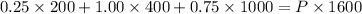
We get, Total pressure, P = 0.75 atm