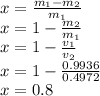Answer:
x=0.8
Step-by-step explanation:
Defining our values we have
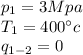
Where
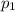 is the pressure of the super heated steam
is the pressure of the super heated steam
 is the Temperature of the super heated steam
is the Temperature of the super heated steam
and
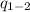 is the pressure in an adiabatic process.
is the pressure in an adiabatic process.
State 1
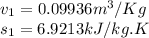
State 2
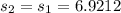
Note that here in state 2 the process is Reversible.
At the value where
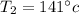 we have
we have
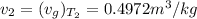
Through this values we can calculate the Fraction of steam,