Step-by-step explanation:
(a)
The relation between frequency and wavelength is shown below as:
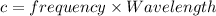
c is the speed of light having value
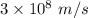
Given, Wavelength = 505 nm
Also, 1 m =
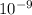 nm
nm
So,
Wavelength =
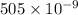 m
m
Thus, Frequency is:
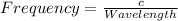
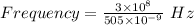
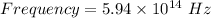
(b)
Also,
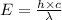
Where,
h is Plank's constant having value
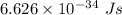
c is the speed of light having value
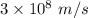
So,
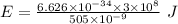
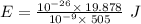
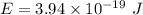
(c)
Electromagnetic spectrum is range of the frequencies and their respective wavelengths of the various type of the electromagnetic radiation.
In order of the increasing frequency and the photon energy and the decreasing wavelength the spectrum are:
Red , Orange, Yellow, Green, Blue, Indigo, Violet
Increasing wavelength is the opposite trend. Thus, The longest visible wavelength is red and the shortest is violet.