Answer:
pH = 6,951
Step-by-step explanation:
The neutral form of aspartic acid is in its isolectric point. For aspartic acid the isoelectric point is the average of pka1 and pka2, thus:
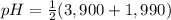 = 2,945
= 2,945
The addition of stoichiometric amounts of NaOH at the first equivalence point will increase the pH at average of pka2 and pka3 thus:
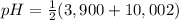 = 6,951
= 6,951
Thus, pH at the first equivalence point is 6,951
I hope it helps!