Answer:
a)mass flow=6lbm/s
b)the temperature at the compressor exit= 1269◦F
Step-by-step explanation:
Hello!
To solve this problem we will perform the following steps
1.Use thermodynamic tables to find the density and entalpy of the air using the properties of temperature and pressure (2 psia and 77.3 ° F)
Density = 0.06032 lbm / ft ^ 3
h1=entalpy=351.2Btu/lbm
2.
Calculate the mass flow by multiplying the flow by the density, remember to apply conversion factors
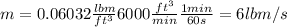
3.We apply first law of thermodynamics in the compressor, it establishes that the energy that enters a system is the same that must come out.
Energies entering the compressor =
-Electric power (800hp)
=W=565.65btu/s
-flow energy (mh1)
energies coming out of the compressor
-heath= (Q)(m
)
-flow energy (mh2)
applying the above we have the following energy balance equation
W+mh1=Qm+mh2
Our goal is to determine the value of the enthalpy of output h2 in order to find the air temperature using thermodynamic tables
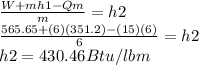
4.finally we use thermodynamic tables to find the temperature using enthalpy
T2=1269◦F