Step-by-step explanation:
The given data is as follows.
Heat capacity = 41.70 kJ/K,
Temperature change =
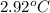
Hence, calculate the quantity of heat as follows.
Quantity of heat = heat capacity × temp. change
= 41.70 kJ/K × 2.92 K
= 121.764 kJ
This heat energy is related to burning of 3.80 g of given sample.
Therefore, calculate the heat per gram as follows.
Heat =
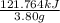
= 32.04 kJ/g
As, 1 nutritional calorie = 1000 calorie
1 calorie = 4.2 J
Therefore, calculate the heat in terms of calorie as follows.
Heat =
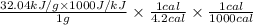
= 7.62 calorie
Thus, we can conclude that there are 7.62 calories per gram of the candy.