Answer: 0.0746 grams of hydrogen
Step-by-step explanation:
To calculate the mass of hydrogen :
(Vapor pressure of water is 23.78 mmHg at 25 ∘C )
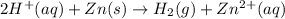
According to avogadro's law, 1 mole of every substance occupies 22.4 L at NTP, weighs equal to the molecular mass and contains avogadro's number
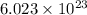 of particles.
of particles.
According to the ideal gas equation:

P = Total Pressure = 752 mm Hg
pressure of hydrogen = Total Pressure - Vapor pressure of water = (752 -23.78 )mm Hg = 728.22 mm Hg = 0.958 atm (760mmHg=1atm)
V= Volume of the gas = 0.953 L
T= Temperature of the gas = 25°C = 298 K
R= Gas constant = 0.0821 atmL/K mol
n= moles of gas= ?
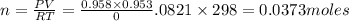
Mass of
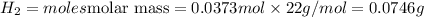
Thus 0.0746 grams of hydrogen is collected.