Answer:
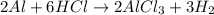
5.39 g
Step-by-step explanation:
The balanced equation for the reaction of aluminum with hydrochloric acid is shown below as:-
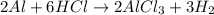
Given,
Pressure = 0.750 atm
Temperature = 30.0 °C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T = (30.0 + 273.15) K = 303.15 K
T = 303.15 K
Volume = 10.0 L
Using ideal gas equation as:
PV=nRT
where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 0.0821 L.atm/K.mol
Applying the equation as:
0.750 atm × 10.0 L = n × 0.0821 L.atm/K.mol × 303.15 K
⇒n = 0.3013 moles
Moles of hydrogen obtained = 0.3013 moles
From the reaction,
3 moles of hydrogen gas are furnished when 2 moles of aluminum is consumed.
Also,
1 mole of hydrogen gas are furnished when
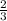 mole of aluminum is consumed.
mole of aluminum is consumed.
Thus,
0.3013 mole of hydrogen gas are furnished when
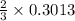 mole of aluminum is consumed.
mole of aluminum is consumed.
Moles of aluminum consumed = 0.2 moles
Also, Molar mass of aluminum = 26.981539 g/mol
So, Mass = Moles*Molar mass = 0.2 moles*26.981539 g/mol = 5.39 g