Answer: 404.04 kJ.
Step-by-step explanation:
To calculate the moles, we use the equation:
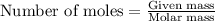
moles of
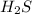
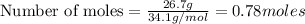
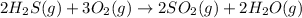
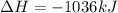
According to stoichiometry :
2 moles of
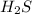 on burning produces = 1036 kJ
on burning produces = 1036 kJ
Thus 0.78 moles of
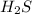 on burning produces =
on burning produces =
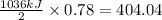
Thus the enthalpy change when burning 26.7 g of hydrogen sulfide is 404.04 kJ.