Step-by-step explanation:
Given that,
The mean kinetic energy of the emitted electron,
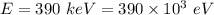
(a) The relation between the kinetic energy and the De Broglie wavelength is given by :
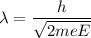
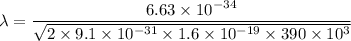
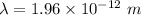
(b) According to Bragg's law,
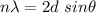
n = 1
For nickel,
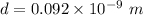
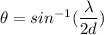
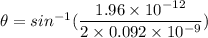
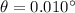
As the angle made is very small, so such an electron is not useful in a Davisson-Germer type scattering experiment.