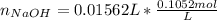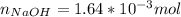Answer:
Moles of NaOH that reacted:
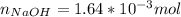
Step-by-step explanation:
During the titration, all moles of NaOH added in the solution, react with the acetylsalicylic acid neutralizing each other.
The titration required 15.62 ml (0.01562 L) of the 0.1052 M NaOH solution. The moles of NaOH in that volume are: