Answer:
Opiton a) t-butanol
Step-by-step explanation:
The boiling point is a good property to identify substances because it is charateristic of each substance. The density is also characteristic of each one but its dependence of the temperature could lead to wrong conclusions if you don´t measure right the room temperature.
Being that said, once calculated the boiling point, in tables you will find the boiling point of many substances. By looking the value you obtained you can determine which substance it is.
So:
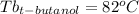
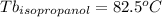
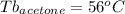
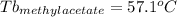
As can be seen, the unknown substance is t-butanol