If the vapor pressure of water (
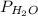
) at 20.0°C is 17.5 mm Hg, and the atmospheric pressure measured by a barometer (
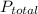
) was 757.3 mm Hg, what is the partial pressure of H₂ gas (
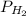
) in the gas mixture in a buret? (Use Dalton’s Law of Partial Pressures to solve for
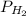
)