Answer:
The mass of the silver block is 95.52 grams.
Step-by-step explanation:
Heat lost by silver will be equal to heat gained by the water
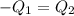
Mass of silver=
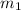
Specific heat capacity of silver =
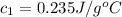
Initial temperature of the silver =
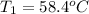
Final temperature of a silver =
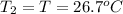 =
=
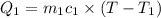
Mass of water=
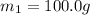
Specific heat capacity of water=
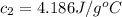
Initial temperature of the water =
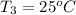
Final temperature of water =
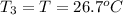
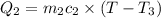
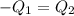
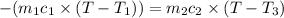
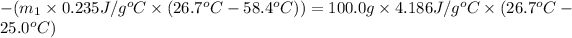
On substituting all values:
we get,
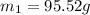
The mass of the silver block is 95.52 grams.