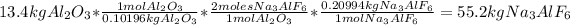Answer:
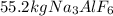
Step-by-step explanation:
1. First balance the equation for the synthesis of cryolite:
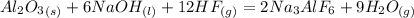
2. Find the limiting reagent between the
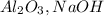 and
and
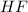
- First calculate the number of moles of each compound using its molar mass and the mass that reacted completely:
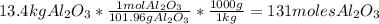
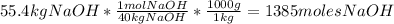
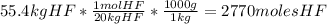
- Divide the number of moles obtained between the stoichiometric coefficient of each compound in the chemical reaction:
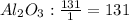
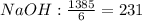
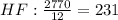
The
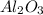 is the limiting reagent because it has the smallest number.
is the limiting reagent because it has the smallest number.
3. Find the mass of cryolite produced: