Answer:
pH 9,8 is likely to work best for this separation
Step-by-step explanation:
Ion exchange chromatography is a chemical process where molecules are separated by affinity to an ion exchange resin. To separate different aminoacids you must use the isoelectric point (That is the pH where the aminoacid will be in its neutral form).
For lysine, PI is:
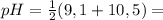 9,8
9,8
For arginine:
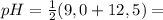 10,75
10,75
At pH = 9,8 lysine will be in its neutral form and will not be retain in the column but arginine will be in +1 charge being retained by the ion exchange resin.
Thus, pH 9,8 is likely to work best for this separation
I hope it helps!