Answer:
the specific heat capacity of the alloy in J/(g.K) is
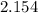
Step-by-step explanation:
Knowns
Final temperature
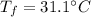 , mass of water
, mass of water
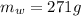 , specific heat capacity of water
, specific heat capacity of water
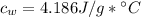 , initial temperature of water
, initial temperature of water
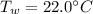 , mass of the alloy
, mass of the alloy
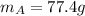 and initial temperature of the alloy
and initial temperature of the alloy
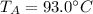 .
.
Applying Formula
We are going to use the following formula
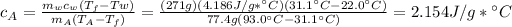
Finally in this case, as the specific heat capacity is measured grade per grade, conversion between Celsius and Kelvin is direct 1:1