Answer:
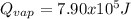
Step-by-step explanation:
Hello!
In this case, since this is the vaporization process of water, we need to keep in mind that the enthalpy of vaporization of this compound is 2.256 kJ/g, for 350 g, we obtain:
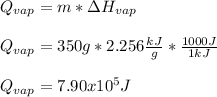
Because the process is carried out at 100 °C, the boiling temperature of water, already.
Best regards!