Step-by-step explanation:
According to the reaction, 2 moles of
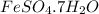 =
=
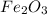
Molar mass of
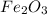 is 159.69 g/mol and mass is given as 0.201 g.
is 159.69 g/mol and mass is given as 0.201 g.
Therefore, calculate the number of moles of
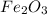 as follows.
as follows.
No. of moles =
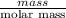
=
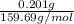
=
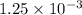 mol
mol
Hence, moles of
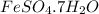 = 2 × moles of
= 2 × moles of
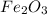
Therefore, moles of
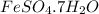 =
=
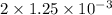 mol
mol
= 0.0025 mol
Now, calculate the mass of
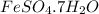 as follows.
as follows.
No. of moles =
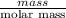
mass = no. of moles × molar mass of
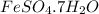
=
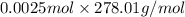
= 0.695 g
Thus, we can conclude that 0.695 g is the mass of
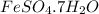 in 2.955 g of the given sample.
in 2.955 g of the given sample.