a) For the thermal efficiency we have
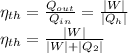
With the previously values we know that
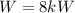 and
and
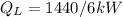 (convert the min to sec)
(convert the min to sec)
Replacing the values
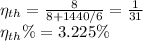
b) We use the formula of carnot efficiency
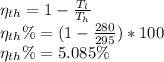
**Note that apply the formula of carnot cycle we need to consider that there is no exchange of heat, there is no friction and the reservior are completely insulated