Answer:
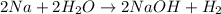
Step-by-step explanation:
Redox reaction is a reaction in which both oxidation and reduction reactions are carried out.
In given reactions, if we observe the reaction that indicating redox reaction is
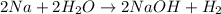
This is because in this reaction oxidation number of sodium is increased from
 . And thus it is oxidized while the oxidation number of hydrogen is decreased from
. And thus it is oxidized while the oxidation number of hydrogen is decreased from

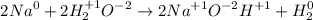
Hence this reaction is redox reaction