Answer:
a. 3,6x10⁻⁴ moles
b. 1,096M
c. 1,53 g of NaOH
Step-by-step explanation:
a. Theobromine has a molar mass of:
C: 12,01×7= 84,07 g/mol
H: 1,01×8= 8,08 g/mol
N: 14×4= 56,04 g/mol
O: 16×2= 32 g/mol
84,07 g/mol + 8,08 g/mol + 56 g/mol + 32 g/mol = 180,15g/mol. Thus, in 0,064g of theobromine are:
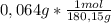 = 3,6x10⁻⁴ moles -two significant figures-
= 3,6x10⁻⁴ moles -two significant figures-
b. Molar mass of H₂SO₄ is 98,079 g/mol.
151,1g are:
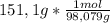 = 1,54 moles of H₂SO₄
= 1,54 moles of H₂SO₄
As molarity is moles of solute per liters of solution, molarity is:
1,54 moles of H₂SO₄ / 1,405L = 1,096 M
c. 2,30x10²² molecules of NaOH are:
2,30x10²²molecules × (1mol/6,022x10²³molecules) = 0,0382 moles of NaOH
As molar mass of NaOH is 40,04g/mol:
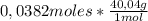 = 1,53 g of NaOH
= 1,53 g of NaOH
I hope it helps!