Answer:
85.85 %
Step-by-step explanation:
The formula for the calculation of moles is shown below:

Given: For propylene
Given mass = 84 g
Molar mass of propylene = 42 g/mol
Moles of propylene = 84 g / 42 g/mol = 2 moles
Given: For ammonia
Given mass = 34 g
Molar mass of ammonia = 17 g/mol
Moles of ammonia = 34 g / 17 g/mol = 2 moles
According to the given reaction:
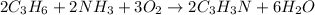
2 moles of ammonia react with 2 moles of propylene to give 2 moles of acrylonitrile.
Thus, Moles of acrylonitrile = 2 Moles
Molar mass of acrylonitrile = 53 g/mol
Mass of acrylonitrile = Moles × Molar mass = 2 × 53 g = 106 g
Theoretical yield = 106 g
Given experimental yield = 91 g
% yield = (Experimental yield / Theoretical yield) × 100 = (91/106) × 100 = 85.85 %